ORACOL® (S10) - Saliva Collection Device
ORACOL® Saliva Collection Device Product Code / Catalogue Number: S10
ORACOL® Product Code / Catalogue Number: S10
WHAT ARE THE BENEFITS?

Simple & Easy to Use

Gingival Crevicular Fluid Enriched Sample

High quality yield of saliva (Vyse et al. 2001)

1 - 2 minute collection time

Non-invasive alternative to blood tests

Cost Effective
WHAT HAS IT BEEN USED FOR?

RNA/DNA Testing

Hepatitis A & B

Measles, Mumps & Rubella (MMR)

Visceral Leishmania

HIV / AIDS

Ebola

COVID19 Antibody Testing

Syphilis
PRODUCT INFORMATION:
|
Product Code: |
S10 |
|
Box Quantity: |
500 units |
|
Capacity: |
> 200µL |
|
Features: |
CE Marked | FDA Registered | Sterile |
|
Instructions for Use: |
|
|
Processing Guide: |
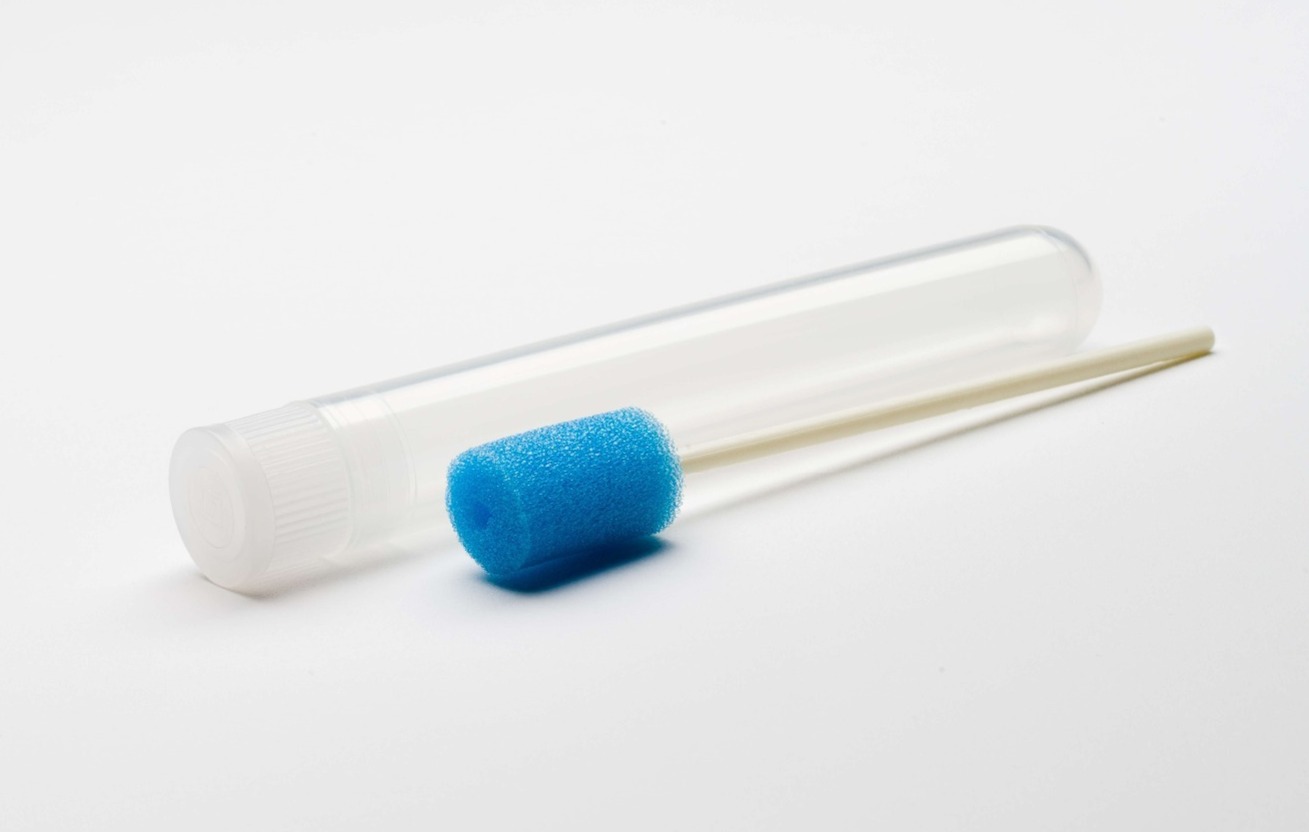
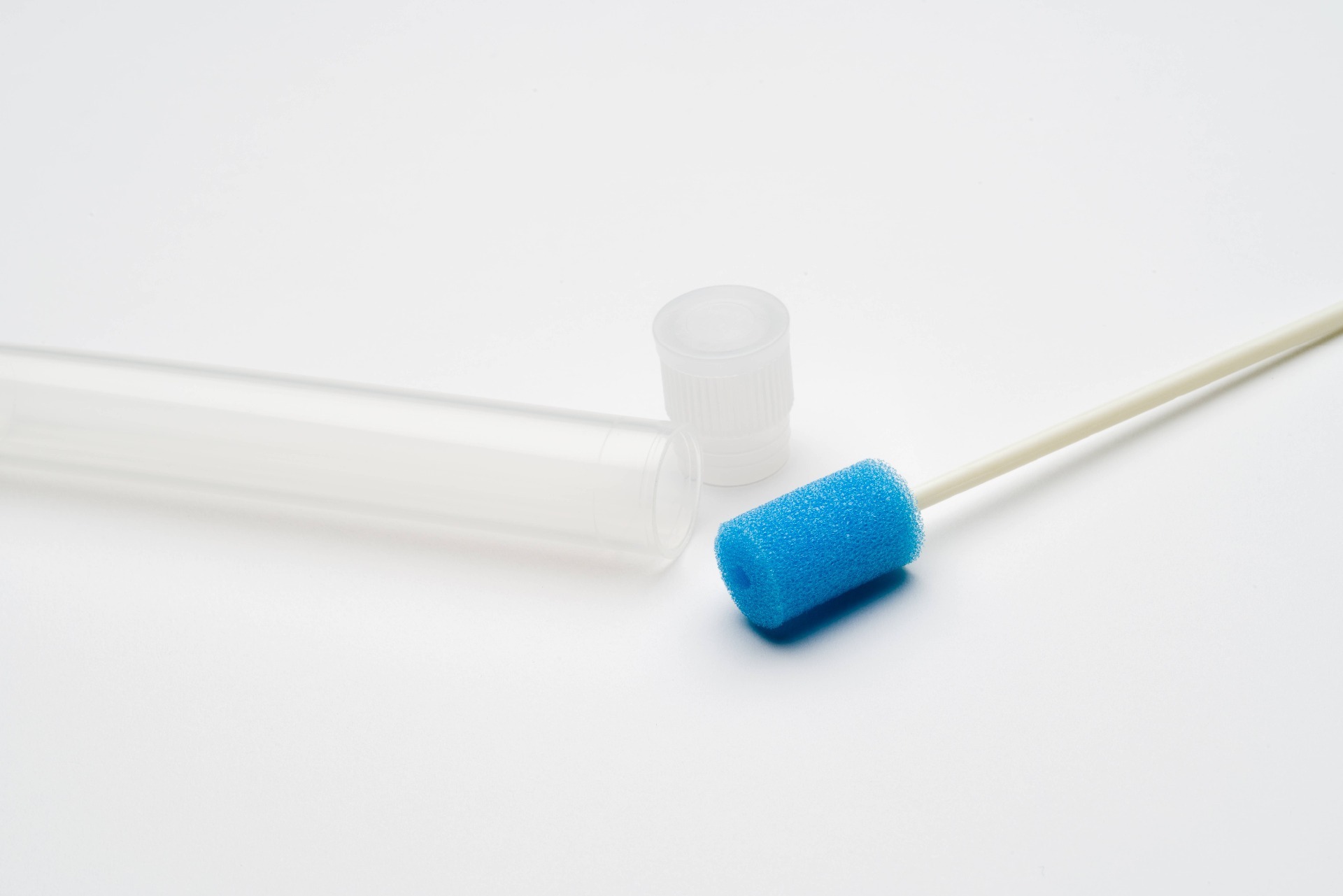
The ORACOL® is a simple yet effective device for the collection of Oral Fluid. Originally designed for the Public Health Laboratory in England over 30 years ago, the ORACOL® has an established history within the scientific community of being a reliable method of saliva collection for testing the presence of a range of pathogens.
Our device is non-invasive and user friendly, making the acquisition of saliva via the foam swab a simple process.
Designed to be used in a similar way to a toothbrush, saliva is collected by rubbing the soft but abrasive sponge swab firmly along the gumline.
The unique texture of the swab encourages the extraction of gingival crevicular fluid (GCF contains high IgG concentration).
The ORACOL® is used worldwide to aid in testing both adults and children (paediatrics), and therefore makes the collection of saliva a favoured alternative to blood tests and nasopharyngeal swabs.


WHAT ARE THE BENEFITS?

Simple & Easy to Use

Gingival Crevicular Fluid Enriched Sample

1 - 2 minute collection time

Non-invasive alternative to blood tests

Cost Effective
WHAT HAS IT BEEN USED FOR?

RNA/DNA Testing

Measles, Mumps & Rubella (MMR)

HIV / AIDS

Ebola

COVID19 Antibody Testing
PRODUCT INFORMATION:
|
Box Qty: |
500 units |
|
Capacity: |
> 200µL |
|
Features: |
CE Marked FDA Registered Sterile Single Use |
|
Instructions: |
The ORACOL® is a simple yet effective device for the collection of Oral Fluid. Originally designed for the Public Health Laboratory in England over 30 years ago, the ORACOL® has an established history within the scientific community of being a reliable method of saliva collection for testing the presence of a range of pathogens.
DISCLAIMER:
Malvern Medical Developments Ltd can not process or conduct any testing on saliva samples taken with this device. This should be conducted by your own research laboratory / similar.


WHAT ARE THE BENEFITS?

Simple & Easy to Use

Gingival Crevicular Fluid Enriched Sample

High quality yield of saliva (Vyse et al. 2001)

1 - 2 minute collection time

Non-invasive alternative to blood tests

Cost Effective
WHAT HAS IT BEEN USED FOR?

RNA/DNA Testing

Hepatitis A & B

Measles, Mumps & Rubella (MMR)

Visceral Leishmania

HIV / AIDS

Ebola

COVID19 Antibody Testing

Syphilis
PRODUCT INFORMATION:
|
Product Code: |
S10 |
|
Box Quantity: |
500 units |
|
Capacity: |
> 200µL |
|
Features: |
CE Marked | FDA Registered | Sterile | Single Use |
|
Instructions for Use: |
|
|
Processing Guide: |

The ORACOL® is a simple yet effective device for the collection of Oral Fluid. Originally designed for the Public Health Laboratory in England over 30 years ago, the ORACOL® has an established history within the scientific community of being a reliable method of saliva collection for testing the presence of a range of pathogens.
Our device is non-invasive and user friendly, making the acquisition of saliva via the foam swab a simple process
The unique texture of the swab encourages the extraction of gingival crevicular fluid (GCF contains high IgG concentration).
The ORACOL® is used worldwide to aid in testing both adults and children (paediatrics), and therefore makes the collection of saliva a favoured alternative to blood tests and nasopharyngeal swabs.
DISCLAIMER:
Malvern Medical Developments Ltd can not process or conduct any testing on saliva samples taken with this device. This should be conducted by your own research laboratory / similar.












